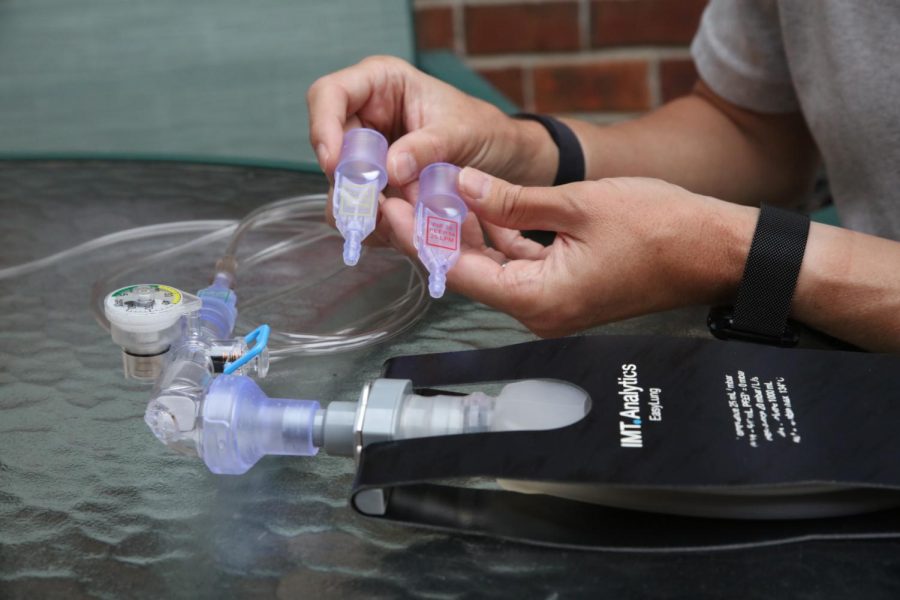
An idea born out of the ventilator shortages from the COVID-19 pandemic is onto the next step to possibly being approved for widespread medical use.
The idea is a 3D-printed “ARMEE” — Automatic Respiration Management Exclusively for Emergencies — ventilator, in part developed by Dr. Brian Froelke, an Oakville physician who works in the trauma center of a major research university in St. Louis, and is the chief medical officer of fluidIQ, a start-up MedTech company developing fluidics-based respiratory solutions.
The ventilator is essentially a reversed-engineered 1960s-era basic ventilator developed by the U.S. Army, small enough to fit into a pocket. It has no moving parts or electricity and is the size of a lipstick tube.
In October, the Science Translational Medicine journal shared news of positive study results of large-animal testing with the 3D-printed ventilator, performed by the National Institutes of Health. Three different versions of the ventilator were tested on swine to correspond to mild, moderate and severe lung injury, and showed adequate support for acute and mild lung injuries.
“My hope is that this will change the way that we provide ventilation or respiratory assistance in patients in the acute setting such as emergency medicine,” Froelke said in an interview with The Call. “The technology both in its current form as well as future form has a lot of opportunity to miniaturize and improve the respiratory care.”
The company is currently in the process of applying with the Food and Drug Administration to begin human trials and will continue to work with the NIH as well on human studies. Froelke said they are also seeking feedback from partners in the field, such as emergency medical service providers and other subject experts on the application of the ventilator in real-life settings.
“That’s going to be one of the big hurdles and big steps,” Froelke said of the FDA process, which he hopes will begin in spring 2023.
“We have designed this tool to provide lung-protective ventilation and we know that there are a lot of current practices that cause harm to lungs through no fault of anybody’s other than the fact that its difficult to apply just the right amount of pressure and just the right amount of volume,” Froelke said. “This particular device is designed to protect the lungs in the volume and pressure that it provides.”
Right now, Froelke and the rest of the team around the ventilator’s development are looking at its application in circumstances such as emergency and disaster response. Froelke is also the chief medical officer and treasurer of Interstate Disaster Medical Collaborative, a nonprofit organization that provides support for regional disaster medical teams in disasters such as the 2011 Joplin tornado.
“Hazardous materials is another area where this could be of great value. Talking with global toxicology experts, this being a one-use, disposable product allows individuals to go through the decontamination process without contaminating the ventilators on either side of the process,” Froelke said. “It opens up the door to rapid decontamination of patients in those events.”
Froelke said there is also a number of non-governmental organizations and other disaster-response medical teams that are interested in the ventilator’s development.
“Over in the medical and humanitarian response to Ukraine, we have a number of non-governmental organizations and disaster-type of teams that are providing services over there … they have reached out asking about the availability of this product and when it might be available for those providers there,” Froelke said. “A great example would be the short-term evacuation of patients who are on ventilators and can’t be removed safely.”
With the next steps hopefully set to begin next spring, initial FDA testing will be done on people who weigh at least 40 kilograms, or roughly 88 pounds, or greater, although pediatric uses are also being developed.
“Any opportunity to miniaturize something and decrease the need for constant upkeep is a great benefit,” Froelke said. “It’s exciting to be a part of this.”